Abstract
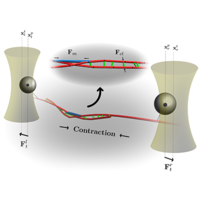
Current understanding of how contractility emerges in disordered actomyosin networks of nonmuscle cells is still largely based on the intuition derived from earlier works on muscle contractility. In addition, in disordered networks, passive cross-linkers have been hypothesized to percolate force chains in the network, hence, establishing large-scale connectivity between local contractile clusters. This view, however, largely overlooks the free energy of cross-linker binding at the microscale, which, even in the absence of active fluctuations, provides a thermodynamic drive towards highly overlapping filamentous states. In this work, we use stochastic simulations and mean-field theory to shed light on the dynamics of a single actomyosin force dipole—a pair of antiparallel actin filaments interacting with active myosin II motors and passive cross-linkers. We first show that while passive cross-linking without motor activity can produce significant contraction between a pair of actin filaments, driven by thermodynamic favorability of cross-linker binding, a sharp onset of kinetic arrest exists at large cross-link binding energies, greatly diminishing the effectiveness of this contractility mechanism. Then, when considering an active force dipole containing nonmuscle myosin II, we find that cross-linkers can also serve as a structural ratchet when the motor dissociates stochastically from the actin filaments, resulting in significant force amplification when both molecules are present. Our results provide predictions of how actomyosin force dipoles behave at the molecular level with respect to filament boundary conditions, passive cross-linking, and motor activity, which can explicitly be tested using an optical trapping experiment.
- Received 15 June 2017
- Revised 5 December 2017
DOI:https://doi.org/10.1103/PhysRevX.8.021006
Published by the American Physical Society under the terms of the Creative Commons Attribution 4.0 International license. Further distribution of this work must maintain attribution to the author(s) and the published article’s title, journal citation, and DOI.
Published by the American Physical Society
Physics Subject Headings (PhySH)
Popular Summary
Biological tissues have the amazing ability to generate significant force and respond to mechanical stimuli. In eukaryotic muscle cells, for example, neural activity can signal actomyosin fibers (long threads of proteins) to contract, generating tension from within the cell’s cytoskeleton that leads to muscle contraction. While nonmuscle cells can also produce significant contractile force with analogous sets of protein ingredients, this somehow takes place even in the absence of well-organized cytoskeletal structures that are thought to be key in generating contractility in muscle cells. This raises the question of whether the same chemical and physical principles are responsible for the contractile behavior and overall microscopic dynamics of nonmuscle cells. This question underlies understanding phenomena such as immune cell activation, wound healing, and cancer metastasis. Using theoretical analyses and computer simulations, we provide a novel explanation as to how contractile forces can be generated within general actomyosin networks with disordered architectures.
First, we show that fundamental contractile elements in actomyosin networks, called force dipoles, harness the thermodynamic free energy of an auxiliary cross-linking protein binding, independent of motor processes. Surprisingly, we discover that both energy- and nonenergy-consuming proteins in tandem can greatly amplify forces at a microscopic level because of a structural ratcheting of the motor by cross-linking proteins. Our work explains this synergistic phenomenon and provides scaling laws for how this force generation depends on the thermodynamic properties of the proteins, pointing to a nonequilibrium ratcheting mechanism as an important requirement for significant contractile force generation in nonmuscle actomyosin systems.
Having worked out the fundamental principles behind elementary actomyosin force dipoles, next we study how a large collection of such dipoles form mesoscopic-scale networks, which architecturally remodel themselves and show interesting, novel contractile behaviors at larger scales.