Abstract
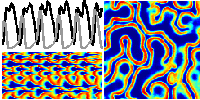
Electrical recordings of brain activity during the transition from wake to anesthetic coma show temporal and spectral alterations that are correlated with gross changes in the underlying brain state. Entry into anesthetic unconsciousness is signposted by the emergence of large, slow oscillations of electrical activity () similar to the slow waves observed in natural sleep. Here we present a two-dimensional mean-field model of the cortex in which slow spatiotemporal oscillations arise spontaneously through a Turing (spatial) symmetry-breaking bifurcation that is modulated by a Hopf (temporal) instability. In our model, populations of neurons are densely interlinked by chemical synapses, and by interneuronal gap junctions represented as an inhibitory diffusive coupling. To demonstrate cortical behavior over a wide range of distinct brain states, we explore model dynamics in the vicinity of a general-anesthetic-induced transition from “wake” to “coma.” In this region, the system is poised at a codimension-2 point where competing Turing and Hopf instabilities coexist. We model anesthesia as a moderate reduction in inhibitory diffusion, paired with an increase in inhibitory postsynaptic response, producing a coma state that is characterized by emergent low-frequency oscillations whose dynamics is chaotic in time and space. The effect of long-range axonal white-matter connectivity is probed with the inclusion of a single idealized point-to-point connection. We find that the additional excitation from the long-range connection can provoke seizurelike bursts of cortical activity when inhibitory diffusion is weak, but has little impact on an active cortex. Our proposed dynamic mechanism for the origin of anesthetic slow waves complements—and contrasts with—conventional explanations that require cyclic modulation of ion-channel conductances. We postulate that a similar bifurcation mechanism might underpin the slow waves of natural sleep and comment on the possible consequences of chaotic dynamics for memory processing and learning.
5 More- Received 5 October 2012
DOI:https://doi.org/10.1103/PhysRevX.3.021005
This article is available under the terms of the Creative Commons Attribution 3.0 License. Further distribution of this work must maintain attribution to the author(s) and the published article’s title, journal citation, and DOI.
Published by the American Physical Society
Popular Summary
The number of neurons that form the cerebral cortex (part of the gray matter) of a human brain is immense: some on average. The spatiotemporal patterning in the activity of these neurons, as observed in brain imaging studies, is believed to be linked to cognitive functions such as learning, recognition, and speech. But the precise linkage between pattern and function remains elusive and is one of the most hotly debated topics in neuroscience today. The problem is being tackled at many structural levels (from networks of individual neurons to neuronal populations or continuum) and using many scientific approaches (such as electrical recordings and functional magnetic resonance imaging). In this paper, we put forward the idea that many aspects of the spatial patterning observed in imaging studies are the result of spontaneous pattern formation generated from the electrical synaptic coupling, also known as gap junctions, between neurons. Such communication channels have been observed experimentally.
We pursue this idea theoretically, in the context of the wake-to-coma transition induced by general anesthesia. Our model, many ingredients of which have been proposed and investigated before, describes the cortex as an excitable, continuum medium of interconnected neurons that is poised at a special point where a spatial (Turing) instability and a temporal (Hopf) instability can coexist. We suggest that normal functioning in the awake brain requires a delicate balance between two competing pressures: the need to form spatial patterns versus the need to generate global rhythms in the cortex. Small changes in model parameters, which reflect altered neurophysiological conditions, can instigate a rebalancing of these two instabilities. Extreme imbalance in either direction is almost certainly pathological: Frozen activity patterns prevent information transfer, while synchronous oscillations across the whole cortex correspond to seizure.
Our study of the model reveals some interesting findings. If the gap-junction-based inhibitory coupling between neurons is sufficiently strong, spatiotemporally patterned neural activity emerges spontaneously with a temporal rhythm that is controlled by the responsiveness of inhibitory chemical synapses. In particular, the model predicts that introduction of anesthetics to the awake brain—in the form of an increased influence of the inhibitory chemical synapses—leads to the emergence of a turbulent, chaotic pattern of slow-wave activity, the result of a subtle rebalancing between Turing and Hopf influences. Gap-junction-facilitated interneuronal communication, acting across the cortex, is crucial to the emergence of this pattern.
Significantly, the slow-wave oscillation is the defining feature of scalp-measured electroencephalography, both under general anesthesia and during the natural nonREM (nonrapid-eye-movement) sleep. Our proposed dynamic mechanism for the slow-wave oscillation contrasts with conventional explanations for anesthesia-induced slow oscillations that require cyclic modulation of ion-channel conductances in single neurons. We suggest that a Turing-Hopf interaction may also underpin the slow-wave stage of natural sleep.